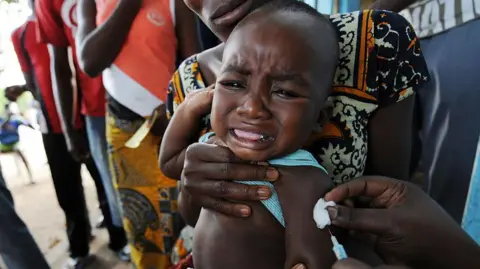
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticized by the World Health Organization as unethical.
The US-funded study had sought to give one set of babies the vaccine at birth, while another would have had the shot delayed until six weeks of age. The WHO said it had significant concerns about the plan, and described the birth-dose vaccine as an effective and essential public health intervention, with a proven record.
The US health department, headed by Robert F. Kennedy Jr., who has questioned the effects of vaccines, had sought to use the trial to answer questions about the jab's broader health effects.
The WHO stated that its concerns regarded the study's scientific justification, ethical safeguards, and consistency with established standards for research involving humans. It emphasized that the jab has been used for more than three decades in over 115 countries, asserting that withholding a proven life-saving intervention exposes newborns to potentially irreversible harm.
Currently, around 14,000 babies in the country were slated to participate in the study funded by the US and led by Danish researchers. However, public outrage led to the timely suspension of the trial by the Guinea-Bissau government.
Critics have questioned why infants in Guinea-Bissau were being selected for this research, especially since the vaccine has a confirmed record of efficacy. The WHO recommends that all newborns receive the hepatitis B vaccine within 24 hours of birth, as infection at birth is the most common path to chronic hepatitis B infection.
With hepatitis B prevalence at alarming rates in Guinea-Bissau, the current administration aims to introduce the birth dose nationwide by 2028 to align with global vaccination standards.