World Health Organization Condemns Controversial US-Funded Vaccine Trial in Guinea-Bissau
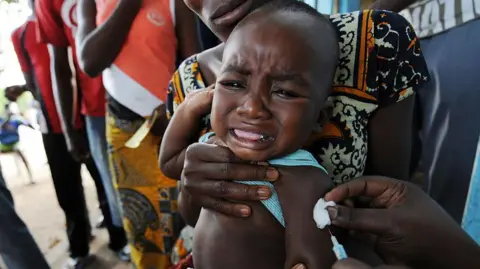
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticised by the World Health Organization as unethical.
The US-funded study planned to administer the vaccine to one group of babies immediately at birth, while delaying the shot for another group until six weeks old. The WHO raised significant concerns regarding the scientific justification and ethical safeguards of the project.
Notably, the US health department, led by vaccine-sceptic Robert F. Kennedy Jr., intended to explore broader health effects of the vaccine through this trial. The WHO emphasized the proven efficacy of the birth-dose vaccine, which has been recognized for over three decades.
The WHO stated that selectively providing a life-saving vaccine to some newborns exposed others to potentially irreversible harm and argued that trials withholding proven treatments are only acceptable when no effective alternative exists.
With more than 12% of Guinea-Bissau's adult population estimated to have chronic hepatitis B, vaccination at birth is critical for preventing the virus’s transmission from mother to child in 70-95% of cases. The WHO recommends that all newborns receive the hepatitis B vaccine within the first 24 hours of birth.
The Guinea-Bissau government suspended the trial in the wake of public outcry, with critics like former health minister Magda Robalo asserting, Guinea-Bissauans are not guinea pigs. Public sentiment against the study intensified following a change in the US vaccination advisory panel, which recently voted to stop recommending universal hepatitis B vaccinations for newborns.
Amidst these controversies, the WHO plans to assist Guinea-Bissau in implementing the birth-dose vaccine by 2028 to align with global health standards, reaffirming the necessity of ethical considerations in clinical research.