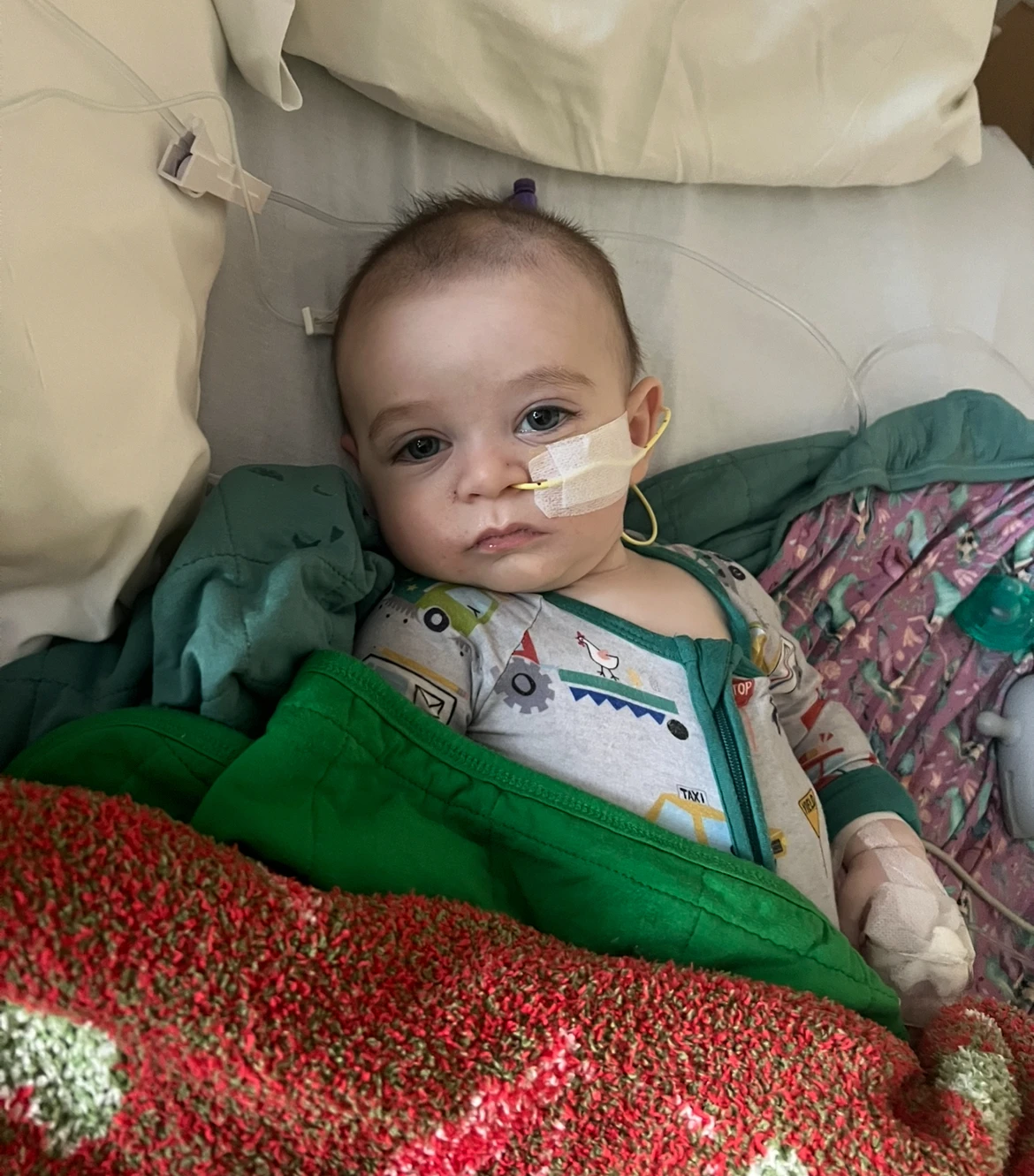
A 10-month-old boy in Portland, Oregon, is currently battling the effects of infant botulism after consuming ByHeart baby formula, which was tainted and donated through a program aimed at assisting low-income families.
Ashaan Carter has been hospitalized due to the dangerous infection that has affected more than 50 infants across the United States. His mother, Angel Carter, received the affected formula just days before a nationwide recall, provided by a state case worker from the Oregon Department of Human Services.
Initially breastfeeding, Angel accepted the formula under the impression it would help her struggling milk supply. I accepted it thinking, ‘OK, I’m hoping my baby can get on a bottle,’ she shared. Unfortunately, this decision soon led to dire health consequences for her son.
State officials confirmed that the ByHeart formula was distributed through nonprofit groups such as the PDX Diaper Bank, one of many involved in the company's 'OpenHearted Initiative' to provide formula to families in need. Since June 2022, nearly 24,000 cans have been distributed, but all products have since been recalled due to possible contamination concerns.
Ashaan’s condition deteriorated rapidly after consuming the formula, as he developed severe constipation and muscle weakness, rendering him largely immobile. He was admitted to Randall Children’s Hospital in Portland, where doctors confirmed the diagnosis of suspected infant botulism linked to the formula.
Undergoing treatment with an IV medication known as BabyBIG, which provides necessary antibodies, Ashaan was hospitalized twice, once for nearly two weeks, but has required ongoing medical care, including the use of a feeding tube due to muscle weakness.
As the situation escalated, the case worker notified Angel to halt use of the product, but the damage was already done. He was just withering away, she recounted, expressing her fear for her son’s survival.
This alarming incident raises significant concerns regarding the safety and reliability of food products distributed to vulnerable families. Dr. Sylvia Peterson-Perry, who has treated Ashaan, emphasized the tragic impact on families who depend on such assistance.
In response, a number of lawsuits have been filed against ByHeart and retailers selling the contaminated formula. Legal action is being taken by families affected, as they seek accountability and justice following this public health crisis.
With no new cases reported since December 17, 2023, the U.S. Food and Drug Administration continues to monitor the situation, while ByHeart’s production has been halted amid ongoing investigations into the source of contamination.