ATLANTA (AP) — Access to COVID-19 shots is a critical topic as Health Secretary Robert F. Kennedy Jr.’s Advisory Committee on Immunization Practices (ACIP) meets again following a controversial vote delay regarding another vaccine for newborns.
Frustrations are mounting among the public as many are struggling to ascertain whether they qualify for the updated COVID-19 vaccines, especially since infections have been on the rise in recent weeks.
Recent restrictions by the FDA have limited the availability of this year’s vaccines from Pfizer, Moderna, and Novavax. Now, ACIP has to decide who should receive these vaccines, decisions that will significantly impact insurance coverage and pharmacy administration across various states.
It remains unclear if the newly formed committee, stocked with members critical of coronavirus vaccinations, will impose further restrictions. Dr. Phil Huang, a family physician and Dallas County health department director, noted the confusion is particularly challenging for low-income families depending on federally funded health programs.
The panel commenced its second day of meetings with unsettled impressions regarding whether a historic CDC recommendation for newborn hepatitis B vaccination should be altered. Health professionals express strong opposition, affirming the vaccine's safety and effectiveness in curbing infant infections.
Last Thursday, the committee advised that children under 4 should receive MMR (measles, mumps, and rubella) and chickenpox vaccines as separate shots rather than the combined MMRV version. Changes will impact how the Vaccines for Children program operates in alignment with the updated guidelines.
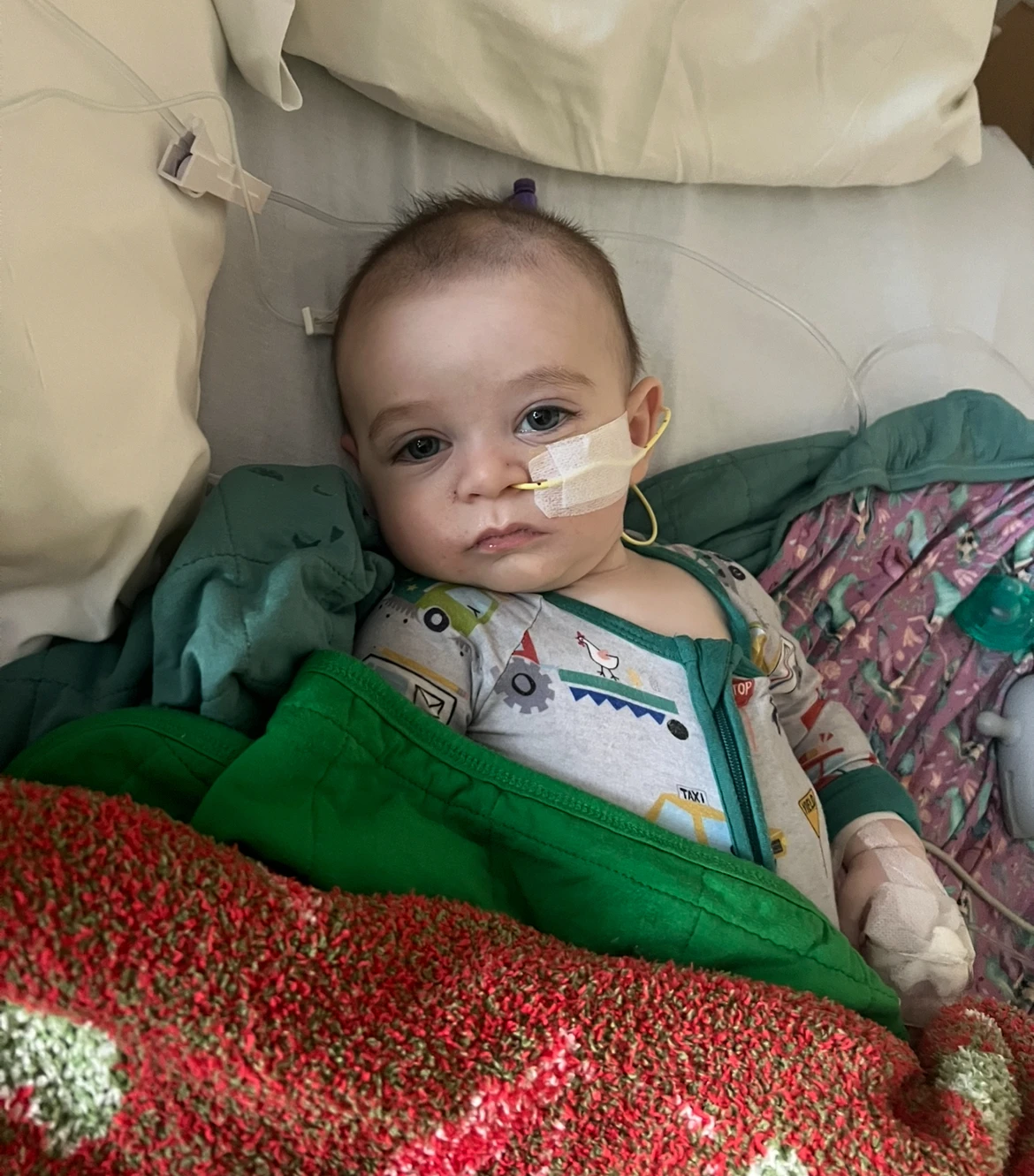
The ongoing pandemic has underscored the importance of COVID-19 vaccinations. CDC data suggests substantial hospitalizations and fatalities among unvaccinated seniors and children. As federal health organizations like the American Academy of Pediatrics advocate for wider access to COVID-19 vaccines, states are implementing measures to ensure availability despite potential limitations from ACIP's decisions.
Health insurers have pledged to cover vaccinations through 2026, attempting to alleviate some public concerns over access.