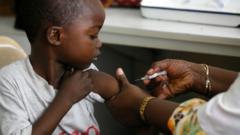
The Swiss authorities have approved Coartem Baby, paving the way for its distribution across Africa within weeks. This new malaria treatment targets the most vulnerable population—babies and young children—offering a crucial medical breakthrough in combating malaria's terrible toll, especially among children under five.
Groundbreaking Malaria Treatment for Infants Approved and Set for Launch
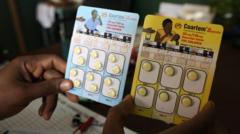
Groundbreaking Malaria Treatment for Infants Approved and Set for Launch
The first-ever malaria medication specifically designed for infants and young children receives approval and will soon be available in African regions heavily impacted by the disease.
The first malaria treatment designed specifically for babies and very young children has received approval, marking a significant advance in the fight against this deadly disease. The new drug, named Coartem Baby, is expected to be rolled out in various African nations in the coming weeks, particularly those with the highest malaria incidence rates.
Prior to this approval, infants needing malaria treatment were administered medications formulated for older children, which posed overdose risks due to differences in physiological responses and developing organ functions in babies. In 2023 alone, malaria claimed approximately 597,000 lives, with a startling 75% of those fatalities occurring in children under five, underscoring the urgent need for age-appropriate treatments.
Manufactured by Novartis, this innovative medication was developed in collaboration with the Medicines for Malaria Venture (MMV), a non-profit organization that includes backing from several governments and global agencies. The company has pledged to distribute it on a largely not-for-profit basis, committing to the equitable access of this essential medication.
Novartis CEO, Vas Narasimhan, highlighted the importance of this breakthrough in combating malaria’s impact on the most vulnerable populations, stating, "For more than three decades, we have stayed the course in the fight against malaria, working relentlessly to deliver scientific breakthroughs where they are needed most."
The approval was also backed by extensive trials in eight African nations, which are expected to be some of the first to utilize this vital new treatment. Martin Fitchet, CEO of MMV, expressed his optimism regarding the approval, stating it is a crucial step toward alleviating the severe burden of malaria in children.
Dr. Marvelle Brown from the University of Hertfordshire emphasized the significance of this development, noting the high mortality rates associated with malarial infections in sub-Saharan Africa, especially for infants born with conditions like sickle cell disease. He asserts that Novartis' commitment to a not-for-profit model can help mitigate inequalities in healthcare access.
As the world cautiously looks forward to deploying Coartem Baby, health experts remain hopeful that it will significantly reduce malaria's grip on the youngest and most at-risk populations.