The approval of Coartem Baby marks a significant milestone in addressing the malaria crisis among infants and young children, particularly in Africa.
First Malaria Treatment for Infants Approved: A Game-Changer in Global Health
First Malaria Treatment for Infants Approved: A Game-Changer in Global Health
Newly approved medicine offers hope in fight against malaria in vulnerable populations.
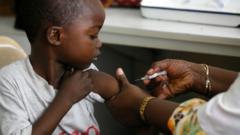
The approval of the world's first malaria treatment designed specifically for infants and very young children is set to transform healthcare responses in malaria-affected regions, particularly in Africa. This groundbreaking medicine is expected to be rolled out within weeks, providing essential care for the youngest and most vulnerable patients suffering from this deadly disease.
Historically, malaria treatments for children have been predominantly formulated for older demographics, posing severe overdose risks for babies, particularly those weighing less than 4.5kg. Current data indicates that malaria is linked to an estimated 597,000 deaths in 2023 alone, with the majority occurring in Africa. Alarmingly, around 75% of these fatalities are children under five, underscoring the critical need for appropriate treatment options in this age group.
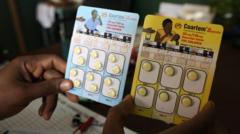
Developed by Novartis, the new pediatric medicine, known as Coartem Baby or Riamet Baby, represents a significant milestone in global health, focusing specifically on infants. This innovative approach aims to fill the existing “treatment gap” and is being introduced in collaboration with the Medicines for Malaria Venture (MMV), which has received support from various governments and foundations.
Vas Narasimhan, Novartis’s CEO, emphasized the importance of this breakthrough, stating that the company has been committed for over three decades to combating malaria. "We are proud to have developed the first clinically proven malaria treatment for newborns and young babies,” he remarked.
Officials from MMV echo this sentiment, calling the introduction of Coartem Baby a vital move towards reducing malaria's significant toll on children's health. Dr. Marvelle Brown from the University of Hertfordshire considers this approval a major step forward in potentially saving innumerable lives among the most vulnerable populations.
The rapid introduction of this crucial medication is anticipated to bolster healthcare efforts in eight African nations that participated in its trials. Notably, public health advocates are optimistic that Novartis's not-for-profit approach will help mitigate healthcare inequalities, enabling greater access to treatment for those in dire need.
As countries continue to grapple with malarial outbreaks, the arrival of Coartem Baby signifies hope in the long-standing battle against one of the world's deadliest diseases, particularly for children facing the harsh realities of malaria.