**The World Health Organization's new agreement mandates data sharing and resource distribution to enhance preparedness for health emergencies.**
**WHO Finalizes Legally Binding Pact to Combat Future Pandemics**

**WHO Finalizes Legally Binding Pact to Combat Future Pandemics**
**New treaty aims to improve global collaboration on health crises after lessons learned from COVID-19.**
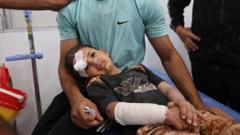
The World Health Organization (WHO) has successfully finalized the terms of a legally binding treaty aimed at enhancing global responses to future pandemics. The agreement, which sought to learn from the chaos and resource competition evident during the COVID-19 pandemic, emphasizes the necessity for streamlined data sharing to enable rapid development of vaccines and treatments.
A notable aspect of the treaty is that the WHO will gain oversight of global supply chains for personal protective equipment (PPE) like masks and medical gowns. WHO Director-General Dr. Tedros Adhanom Ghebreyesus noted the significance of this moment, calling it a "significant milestone" in the global quest for a safer world. He acknowledged the importance of multilateral cooperation, stating that common ground has emerged amidst international divisions.
The treaty, which took three years of negotiations to craft, is historically significant as it marks only the second international agreement in the WHO's 75-year history, the first being a tobacco control treaty signed in 2003. Although the treaty requires formal ratification at the upcoming World Health Assembly, its core principles have been agreed upon.
Notably, the United States did not participate in the final negotiations due to former President Donald Trump's withdrawal from the WHO, meaning the U.S. will not be bound by the treaty when it officially exits in 2026. Under the new agreement, member countries are tasked with guaranteeing the worldwide availability of pandemic-related pharmaceuticals. Agreements stipulate that pharmaceutical manufacturers should allocate at least 10% of their production capacity for vaccines and treatments to WHO, with another 10% provided at affordable rates.
Additionally, provisions allow for technology transfers to lower-income countries under mutually agreed terms to support local production capabilities of vaccines and medications during health crises. This aspect has sparked controversy, given the frustrations developing nations have over vaccine hoarding by wealthier countries during the recent pandemic. Meanwhile, established pharmaceutical nations express concern that mandatory technology transfers could jeopardize ongoing research and development efforts.
At the heart of the treaty is a proposed system for Pathogen Access and Benefit-Sharing (PABS), aimed at facilitating quicker data sharing among pharmaceutical companies to expedite drug development in future outbreaks. This agreement marks a pivotal step in global health collaboration to address shared threats head-on, paving the way for a more coordinated response to upcoming public health emergencies.
A notable aspect of the treaty is that the WHO will gain oversight of global supply chains for personal protective equipment (PPE) like masks and medical gowns. WHO Director-General Dr. Tedros Adhanom Ghebreyesus noted the significance of this moment, calling it a "significant milestone" in the global quest for a safer world. He acknowledged the importance of multilateral cooperation, stating that common ground has emerged amidst international divisions.
The treaty, which took three years of negotiations to craft, is historically significant as it marks only the second international agreement in the WHO's 75-year history, the first being a tobacco control treaty signed in 2003. Although the treaty requires formal ratification at the upcoming World Health Assembly, its core principles have been agreed upon.
Notably, the United States did not participate in the final negotiations due to former President Donald Trump's withdrawal from the WHO, meaning the U.S. will not be bound by the treaty when it officially exits in 2026. Under the new agreement, member countries are tasked with guaranteeing the worldwide availability of pandemic-related pharmaceuticals. Agreements stipulate that pharmaceutical manufacturers should allocate at least 10% of their production capacity for vaccines and treatments to WHO, with another 10% provided at affordable rates.
Additionally, provisions allow for technology transfers to lower-income countries under mutually agreed terms to support local production capabilities of vaccines and medications during health crises. This aspect has sparked controversy, given the frustrations developing nations have over vaccine hoarding by wealthier countries during the recent pandemic. Meanwhile, established pharmaceutical nations express concern that mandatory technology transfers could jeopardize ongoing research and development efforts.
At the heart of the treaty is a proposed system for Pathogen Access and Benefit-Sharing (PABS), aimed at facilitating quicker data sharing among pharmaceutical companies to expedite drug development in future outbreaks. This agreement marks a pivotal step in global health collaboration to address shared threats head-on, paving the way for a more coordinated response to upcoming public health emergencies.