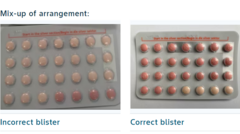
In a troubling development for women relying on hormonal contraception in South Africa, a batch of the widely-used Yaz Plus contraceptive pill has been recalled due to a significant packaging error. The South African Health Products Regulatory Agency announced that the manufacturer, Bayer Ltd, had mistakenly distributed blister packs that contained 24 inactive pills instead of 24 hormone-active pills, raising serious concerns over the method's efficacy.
The affected batch, marked WEW96J and set to expire in March 2026, includes a limited number of packets, prompting immediate action from Bayer and regulatory officials. As a critical precaution, the company has urged women using pills from this batch to halt consumption without delay and consult their healthcare providers.
The standard Yaz Plus pack typically consists of 24 pink hormone-containing pills followed by four light orange hormone-free pills. However, the recalled batch has led to a troubling situation where users may have been led to believe they were taking active pills, while in reality, they were consuming only inactive options, thereby increasing the risk of unintended pregnancies.
Bayer Ltd released a statement underlining that while only a small quantity of this specific batch has been compromised, the nature of the error necessitates caution. "No tablets from these packs shall be used until you have consulted your healthcare practitioner, as they may potentially not provide the contraceptive protection you expect," the notice warned.
Patients who have purchased the affected packets are encouraged to return them to their local pharmacies for replacements or refunds. Moreover, healthcare professionals, including pharmacists and hospitals, have been instructed to return any of the compromised stock.
In light of the incident, Bayer has confirmed that they have identified the root cause of the packaging error and implemented corrective measures to prevent future occurrences. They have also established a helpline for anyone needing additional information regarding the recall or the specific batch. With no other batches implicated, Bayer remains committed to maintaining the integrity of their products.